The question now is what are the odds of another catastrophic disease that decimates the world’s population on the scale of these earlier pandemics?

“It’s impossible to predict,” says virologist and UniSA Adjunct Senior Lecturer Dr TuckWeng Kok, who was a consultant to the World Health Organisation during the SARS (Severe Acute Respiratory Syndrome) outbreak in China in 2002-03.
Dr Kok says prompt identification of the COVID-19 pathogen, the sharing of infection data globally together with significantly improved public health surveillance, monitoring and control have all made a huge difference in limiting or containing the spread of infection.
Improved sanitation, clean water, a better understanding of human biology, containment measures, better medications, antibiotics, antivirals and strong public healthcare systems mean the world is in a better position than it was a century ago when it comes to future outbreaks – but there are no guarantees.

Microbiologist Associate Professor Rietie Venter has personal experience of living through an earlier pandemic. Her husband and her two sons got swine flu in 2009 when she was working at the University of Cambridge.
“I was the only one in the family that didn’t get ill,” Assoc Prof Venter says. “For my family, it was the most ill they’ve ever been. But, even without treatment, everyone survived. In the end, after the outbreak, when they analysed it, fewer people died from swine flu than die from the normal seasonal flu.”
It is estimated that 11–21 per cent of the then global population (of about 6.8 billion), or around 700–1400 million people contracted the illness — more in absolute terms than the Spanish flu pandemic. However, with about 150,000–575,000 fatalities, it had a much lower case fatality rate of 0.01-0.08 per cent.
“There’s a huge scare factor attached to viruses,” Assoc Prof Venter says. “Even if you have a relatively low mortality rate of 2 per cent – which is still very sad, of course – it scares people tremendously.”
Immunologist Dr Maurizio Costabile says there’s a well-established, staged approach for dealing with epidemics – reinforcing the need for good hygiene practices, isolation/containment and then medication/treatment for people who become ill.
“The effectiveness of basic hygiene is underrated by many people,” Dr Costabile says. “There are simple things that can be done that limit the spread effectively … cleaning and washing your hands, wearing masks, sneezing into a tissue or your arm and if you're sick, isolating by staying home.”
While people tend to fear viruses, pandemics can also be caused by bacteria. One of the world’s most devastating pandemics, the Bubonic Plague (or Black Death), was caused by a bacterium carried by rats. The Plague is estimated to have killed up to 200 million people.
The discovery of antibiotics means most bacterial infections can be treated, however misuse of antibiotics has led to the rise of multi-drug resistant bacteria, often called superbugs.
“Over time, the number of antibiotics that have come to market has not increased,” Dr Costabile says. “There was a golden age with penicillin and related antibiotics, but they've lost effectiveness now and they're not being replaced.
“And bacteria have developed resistance – so they're able to persist despite antibiotic treatment.”

UniSA Emeritus Professor Mary Barton says pharmaceutical companies have tended to focus on lifestyle medicines, such as anti-cholesterol drugs and medicines to reduce blood pressure, in recent years because they can be lifelong treatments.
“Whereas you might take an antibiotic for 5-10 days and that’s it … lifestyle drugs are a lot more profitable,” Prof Barton says.
People who stop taking antibiotics when they feel better, rather than completing the prescribed course, are also believed to contribute to antibiotic resistance.
Prof Barton says Australia is high on international league tables for antibiotic use in humans.
“Antibiotics are useless for viral infections like the common cold and should not be used unless really needed but medicos are waking up to unnecessary use,” she says. “If you have a cold or COVID-19, giving antibiotics will not do a thing. You need effective antivirals and there are not that many that are proven to be effective. The problem is that people mistakenly demand antibiotics and go ‘doctor shopping’ to get them.”
She says using antibiotics to treat some infections, such as a mild urinary tract infection, is generally unnecessary, and can lead to antimicrobial resistance.
Assoc Prof Venter says there are countries where up to half of infections with K. pneumoniae (a bacteria found in the normal flora of the mouth, skin, and intestines that can cause destructive changes to human and animal lungs if aspirated) cannot be treated with antibiotics because they’ve become resistant.
“So we already have organisms that are resistant to everything, travelling all over the world, but they rarely reach public attention,” she says.
“It's also more of a financial burden in the sense that if someone goes into hospital for a week, they can end up staying for three months because of an infection; or someone with a minor injury could end up losing an arm.
“Deaths from antibiotic resistance infections are most probably underreported as a lot of the time there will be other contributing factors or comorbidities.”
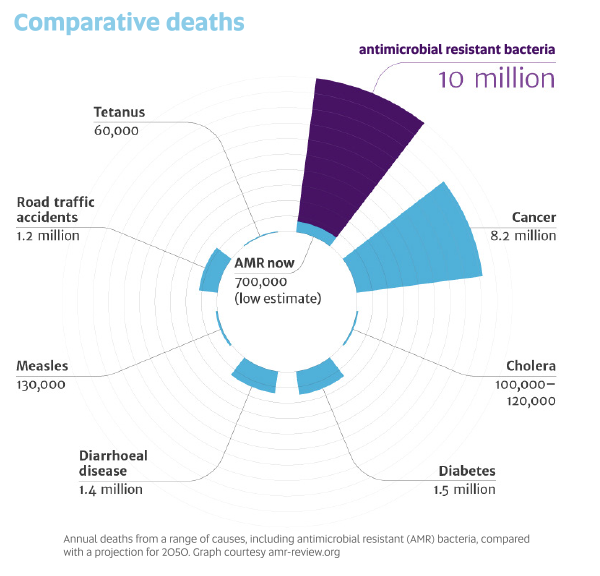
A landmark review into the global problem of antimicrobial resistance warned that if no action is taken, drug-resistant diseases could cause 10 million deaths each year by 2050 and damage the economy at the same catastrophic levels as the 2008-2009 global financial crisis. Today, at least 700,000 people die each year because of drug-resistant diseases. More and more common diseases, including respiratory tract infections, sexually transmitted infections and urinary tract infections, are becoming untreatable.
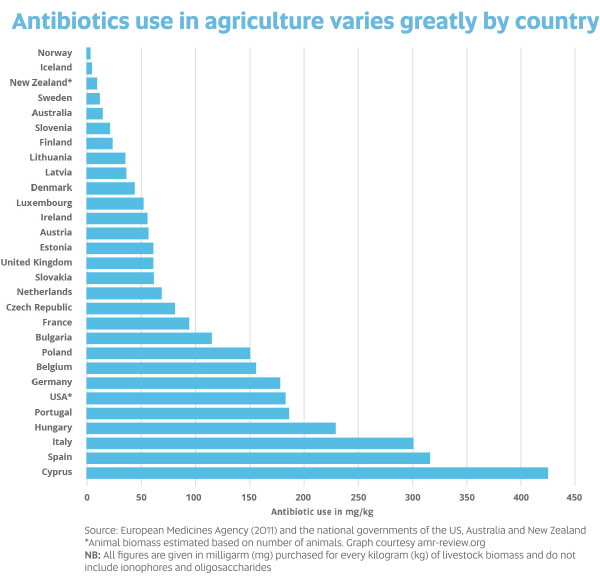
While many people are concerned about antibiotic use in livestock, Prof Barton says this is more of a problem overseas than in Australia because Australia has strict regulations applying to food.
“Overseas there’s a lot more food contaminated with antibiotic resistant bacteria,” Prof Barton says. “Therapeutic antibiotics can no longer be used as growth promotants in Australia.
“Australia needs to keep the pressure on to reduce/control antibiotic use in food-producing animals. However, you can’t forget pets – people have very close contact with their ‘fur babies’ and there is much less control on antibiotic use
There are a small number of instances where bacteria have developed resistance to antibiotics in pets and then transferred to humans.
“Antibiotics of critical importance in human medicine are used in dogs, cats and horses."
Even in 2020, there are ongoing pandemics caused by bacteria but they don’t make headlines.
Tuberculosis kills up to 1.5 million people a year, even though it’s preventable with a childhood vaccine, and normally treatable with antibiotics.
Australia has very low incidence of tuberculosis (TB) but it’s a huge problem among Australia’s neighbours, including Papua New Guinea and Indonesia, where they are dealing with multi-drug resistant TB. Treatment for people with TB that is resistant to antibiotics is longer, more expensive (more than US$1000 per person) and more toxic.
But for now viruses are far more challenging to treat than most bacteria, because each virus needs a specific vaccine or antiviral, based on its properties.
“It's much harder to prepare antivirals because viruses hijack your own machinery, the host’s machinery,” Assoc Prof Venter says.
While much has been made of the early advances towards a vaccine for COVID-19, researchers warn against depending too heavily on this because producing a safe and effective vaccine is a laborious and complex process and does not always deliver results.
“Scientists have been making vaccines for malaria for decades but unfortunately not one has been completely effective,” Dr Costabile says.
Similarly, almost two decades on from the outbreak of the SARS coronavirus (SARS-CoV), there’s no effective vaccine.
Dr Costabile says getting the seasonal flu vaccine remains crucial as some people in Europe have contracted both the flu and COVID-19 at the same time – a double-whammy.
“Antivaxxers have made a sizeable dent into people’s confidence about getting vaccinated. They’ve caused confusion by saying things that have no scientific basis and make no sense,” he says.
“The fact is, three of the biggest advances in medical history have been clean water, antibiotics and vaccines. They've saved millions and millions of lives throughout history. These are scientifically-based, well-founded and well-studied.”
Dr Kok says one of the most interesting aspects of COVID-19 is how it seems to affect the elderly more than children.
“There is an intriguing question as to how it can spread so quickly because all of us have been infected with coronaviruses, the mild ones, and we may not even have known about it, but this is obviously a very new coronavirus – so it has some unusual biological properties.”
All agree basic hygiene, including washing hands, is one of our simplest and strongest weapons against the virus.
MORE DISEASES TO LEAP FROM ANIMALS TO HUMANS
Scientists estimate 75 per cent of new or emerging infectious diseases in people come from animals.
Zoonotic diseases are caused by germs – including viruses, bacteria, parasites and fungi – spread between animals and people.
UniSA Emeritus Professor Mary Barton says human encroachment on animal habitats puts us at greater risk.
“Disease transmission from wildlife species is a problem as humans destroy native animal habitats and force wildlife like bats into closer contact with humans,” she says. “Bats can infect humans directly (eg with diseases such as rabies) but most of the more recent crossovers have been through an intermediate animal host and in the cases of SARS and COVID-19, likely infection of other animals at food markets.”
A species of animal needs to share a common receptor with humans for a virus to transfer directly.
“If animals share the same receptors used by the virus to attach to tissues in the body and start to replicate then infection can move readily from one species of animal to another,” Prof Barton says.
Hendra virus, which can be fatal to humans, is a good example. Scientists believe that fruit bats (flying foxes) are the host of the Hendra virus in Australia and can carry the virus without suffering any ill effects. They excrete the virus in urine. Exactly how it is transmitted from bats to horses is unknown. One theory is that the horse ingests pasture or fruit contaminated with infected bat urine, droppings or saliva. The virus in the horse’s body fluids (including blood, urine, saliva or nasal secretions) can then be transmitted to a person during close contact. There is no evidence of Hendra virus spreading from person to person or from flying foxes to humans.
Similarly, SARS-CoV-2 (the virus that causes COVID-19), is believed to have been transmitted to humans from bats through an intermediary animal. Dozens of people infected early in the outbreak in China worked in a live-animal market in the city of Wuhan.
Associate Professor Rietie Venter says avian flu, swine flu and Middle East respiratory syndrome (MERS) are all examples of viruses that originated in animals.
“Close contact with animals, confined spaces used by animals and humans – such as animal markets, the practice of eating bushmeats in certain cultures, and greater contact with endangered species – I don’t think these things can be ignored as contributing factors,” she says.
But virologist Dr TuckWeng Kok says it’s a two-way street. For example, swine flu was a new strain of H1N1, which first appeared in 1918–1920 as the Spanish flu pandemic. Swine flu is believed to be the result of a re-assortment of bird, swine and human flu viruses.
“The pig is a good melting vessel for flu viruses to produce a new flu strain,” Dr Kok says. “So it’s not just one-way traffic.”

HOW A PANDEMIC TESTS HUMANITY
Fear tends to bring out the best – and worst – in people.

UniSA ethicist Dr Connal Lee says fear in a pandemic can lead to competition for resources.
“Sometimes you can see fear bringing out the best in people – people really want to help their fellow humans,” he says. “It can bring about cooperation. On the other hand, it can bring out competition – there’s not enough goods and people are fighting to get tins of beans in the supermarket.”
That competition can also apply to healthcare, requiring an ethical framework to determine who receives treatment or a vaccine.
Faced with a limited number of beds and staff to care for patients, Italian doctors and health workers faced the excruciating task of having to make decisions about who should get care.
The Italian College of Anesthesia, Analgesia, Resuscitation and Intensive Care published guidelines which prioritised access to intensive care to “those patients with the highest chance of therapeutic success”.
“It's a classic problem of healthcare: Who gets the last bed in a hospital?,” Dr Lee says.
“One approach is aged-based rationing … giving a child a ‘fair innings’. This argument says an infant who is yet to enjoy a ‘fair share of life’, would have a stronger claim to a scarce and vital resource than an elderly person. But it doesn’t specify what a ‘fair share’ of life is and the resulting cut-off point for prioritisation – so this is where things get trickier.
“It really comes down to maximising our use of resources but doing so in a way that is considered fair by society. For example, it would clearly be a poor use of resources to give a vaccine to people who are mortally ill.”
Vaccinations are often prioritised for those considered most at risk of catching a disease or of dying.
But some at-risk groups are stigmatised, making the use of fair principles potentially unpopular.
During the 2009 H1N1 swine flu pandemic in Canada, prisoners were identified as an at-risk group and given priority to be vaccinated in some provinces because of their susceptibility to severe illness and their confined quarters. But after a public outcry, ministers overturned the plan. Obesity was the second most common co-morbidity among Canadian patients admitted to intensive care units with swine flu. However, people with severe obesity were only prioritised for vaccination in some locations. The arguments against these two groups receiving vaccinations tended to relate to personal responsibility and moral blame.
One comment on a CBC News article summarised the argument: “You work out, you ride your bike, you jog and what happens? You are denied the swine flu shot because you’re too healthy. However, the rest of us who sit on the couch, smoke, drink, eat fried foods and basically do nothing, are at the head of the line. How ironic is that?”
What Canadian politicians failed to communicate was that vaccinating at-risk groups before others contributes to the overall health of the community, reducing the number of seriously ill patients who might otherwise tie up crucial resources such as ventilators and intensive care beds. It’s also difficult to prevent a virus spreading from a prison to the wider community.
“Just because allocating vaccines to particular groups can be unpopular is not a sound argument against doing so,” Dr Lee says. “A pandemic is always going to be a test of our humanity. Are we willing to carry the burden of protecting prisoners, obese people or smokers?
“People might say obese people and prisoners get what they deserve and that their predicament is based on their choices but both groups are generally at greater risk of contracting disease. Protecting them protects the health system, which benefits everyone, whereas allowing people to become ill and to be hospitalised is expensive.”
Dr Lee says one of the challenges in a pandemic is that people who catch the disease can also be stigmatised.
“People are both a threat to others and a victim of the disease … you end up with fear of your fellow human beings because they can possibly make you or those close to you sick. Negotiating that fear of each other is tricky.”
The answer, he says, is to try to appreciate – as best you can – what other people might be going through.
“A pandemic is potentially a test of our own ability to respond in the right way for the benefit of our community,” Dr Lee says.
“It’s valuing a willingness to look after those who are most vulnerable – to put yourself in their shoes.
“But it’s hard to envisage yourself in some people’s shoes – for Australians, this might be a person living in Wuhan. It might be someone who’s obese. Increasing our moral imagination will help us to see the point of view of those who are worse off.”
And getting to know people in your community is another crucial way to successfully negotiate a pandemic.
“Is it okay when under duress to trample on strangers to put your family ahead? Communities which pull together, pooling resources and working cooperatively, tend to do better,” he says.
“It’s a lot harder to put me and mine first when you know your neighbours.
“We are better off working together to meet a threat like this one.”